In the medical device manufacturing sector, selecting an appropriate Printed Circuit Board Assembly (PCBA) manufacturer is a critical step in ensuring product quality and safety.
Within the medical device industry, even a small circuit board carries significant responsibility. Whether for pacemakers, medical imaging equipment, or portable monitors, high-performance and highly reliable PCBA lies at the core. Statistics indicate that while China has over 20,000 factories engaged in PCBA SMT assembly and DIP plugin soldering, very few meet the stringent requirements of the medical industry. So, how can one identify a truly suitable partner for medical device manufacturing from the multitude of available manufacturers?

1、Specificity and Importance of Medical PCBA Selection
The quality of medical electronic PCBA directly impacts device performance, safety, and reliability. Unlike consumer electronics, medical devices impose extremely stringent requirements on PCBA:
Medical devices often operate in life-critical scenarios, where even minor failures can lead to severe consequences. Therefore, medical-grade PCBA must possess exceptional stability and fault tolerance.
Medical devices typically have long lifecycles, requiring continuous operation for years or even decades without major failures, which places extreme demands on PCBA durability.
Medical devices must comply with rigorous regulatory standards such as FDA, CE-MDD, and ISO 13485, which cover the entire process from design and development to production and service.
With the trend towards intelligent and miniaturized medical devices, PCBA must accommodate more complex circuit designs while maintaining a compact form factor.
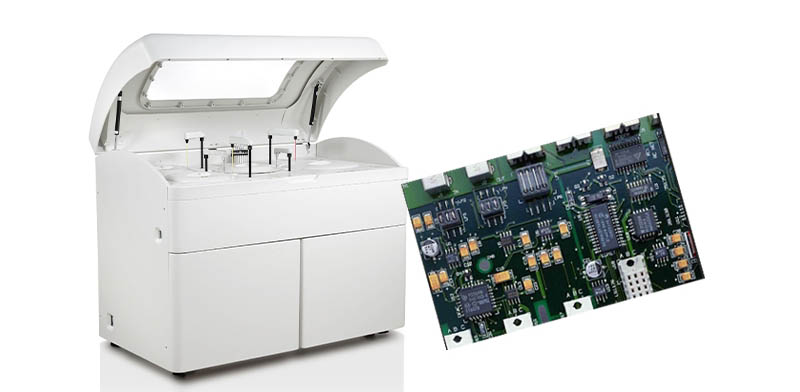
2、Five Core Dimensions for Evaluating Medical PCBA Manufacturers
Certifications: The “Admission Ticket” for the Medical Industry
ISO 13485 certification is the international standard for quality management systems for medical devices and serves as the core credential for medical PCBA manufacturers. This certification specifically addresses design, development, production, and service processes, ensuring full-process quality control.
Beyond ISO 13485, it is essential to verify if the manufacturer holds other certifications such as ISO 9001 (basic quality management) and IATF 16949 (automotive electronics, indicative of high-reliability requirements), which collectively form the foundation of the manufacturer’s quality management system.
Tortai Technologies Co., Ltd., for instance, is certified under the ISO 13485 Medical Device Quality Management System and has established a comprehensive material traceability system for its medical PCBA processing.
Technical Capability: The “Hard Threshold” of Precision Manufacturing
Medical devices frequently utilize ultra-miniature components such as 01005 and 0201, as well as high-density chips like BGA and QFN, demanding extremely high placement accuracy. A qualified medical PCBA manufacturer should achieve placement accuracy within ±0.03mm and be equipped with high-magnification vision alignment systems.
End-to-end inspection capability is crucial: strict control is required at every stage, from solder paste printing (SPI inspection), component placement, and reflow soldering to Automated Optical Inspection (AOI), X-Ray inspection, and functional testing. Particularly for hidden solder joints like those in BGAs, non-destructive testing via 3D X-Ray is mandatory.
Lingzhuo Prototyping employs double-sided SMT assembly technology, mounting components on both sides of the board to enhance integration, thereby meeting the demands for miniaturization and high performance in medical devices.
Quality System: The “Lifeline” of Medical Devices
Medical PCBA manufacturers must implement a robust quality control system encompassing incoming quality control, in-process control, and final product inspection. For critical components such as medical-grade chips and sensors, 100% batch inspection should be performed to ensure compliance with specific requirements like biocompatibility and corrosion resistance.
A traceability system is a distinctive requirement in medical electronics production. Reputable manufacturers assign unique identifiers to each medical PCBA, enabling full data traceability from material procurement and assembly processing to final product testing, thus satisfying medical device regulatory demands.
Tortai Technologies ensures rapid problem localization to specific process steps and clear accountability through comprehensive MES data logging.
Industry Experience: The “Intangible Competitive Edge” in the Medical Field
Successful case histories serve as important references for evaluating a manufacturer’s experience in the medical industry. Priority should be given to manufacturers with proven experience in areas such as patient monitors, imaging equipment (MRI/CT), and wearable medical devices.
Familiarity with medical regulations and standards is also paramount. Since medical devices must comply with international regulations like FDA, CE, and MDD, the PCBA manufacturer’s ability to provide compliance support—such as assisting with documentation preparation and audit responses—is a key consideration.
As a medical electronics PCBA manufacturer, Tortai Technologies’ core strengths are reflected in its full-process, one-stop services, stringent quality control system, and flexible order response capability, making it particularly suited to the high-precision and high-reliability requirements of medical electronics device manufacturers.
Supply Chain and Service: Balancing Efficiency and Reliability
Medical device orders often involve small batches and multiple production runs, requiring PCBA manufacturers to support flexible production. Concurrently, components for medical PCBA frequently face shortage risks; therefore, manufacturers should possess a global procurement network and contingency stocking capabilities.
The value of one-stop services is significant: integrated services spanning PCB design, component procurement, assembly, and testing can reduce communication overhead. Leading manufacturers provide Design for Manufacturability (DFM) analysis to help clients identify and avoid potential process challenges during the design phase, thereby mitigating mass production risks.
Tortai Technologies Co., Ltd. offers comprehensive services covering layout design, PCB fabrication, SMT processing, component sourcing, and BOM fulfillment, with a monthly capacity of up to 6 million points, capable of meeting the production needs of medical device companies across different scales.
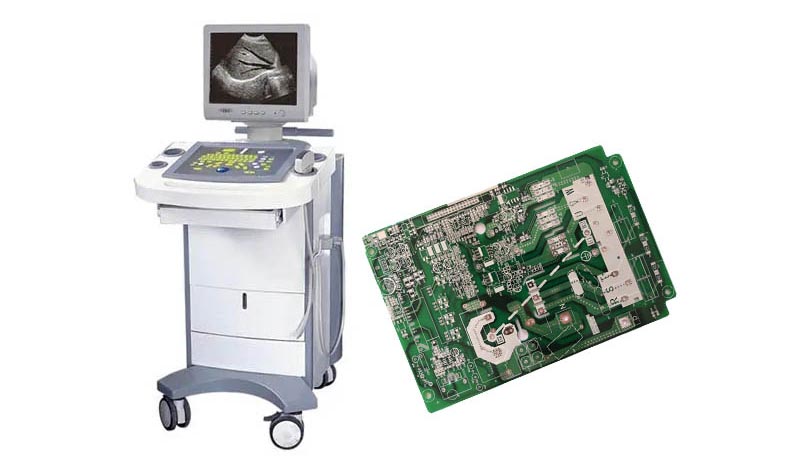
3、On-site Audit: An Indispensable Step
Conducting an on-site audit of the PCBA manufacturer is the most direct method to ensure a correct selection. The audit should focus on the following aspects:
Production Environment: Medical PCBA production typically requires a Class 10,000 cleanroom equipped with anti-static and dust-proof facilities to prevent particulate contamination from affecting device performance.
Equipment Status: Physically inspect the condition and maintenance of placement machines, reflow ovens, and inspection equipment. For example, the Yamaha high-speed placement machines used by Tortai Technologies Co., Ltd. achieve a placement accuracy of ±0.03mm, capable of handling 01005 ultra-miniature components.
Quality Records: Request the manufacturer to present actual quality control records and data, such as Statistical Process Control (SPC) charts and non-conforming product handling procedures, which are vital for assessing their quality assurance capabilities.
Technical Team: Engage with the manufacturer’s engineering team to understand their problem-solving abilities and experience, particularly their depth of understanding regarding the specific requirements of medical devices.

4、Industry Trends and Future Outlook
With the integration of artificial intelligence and IoT technologies, medical devices are evolving towards greater intelligence and remote capabilities, imposing higher demands on PCBA manufacturers:
High-Integration Design: Capability to support complex PCBA designs incorporating AI chips and sensor arrays.
Environmental Compliance and Sustainability: Green manufacturing capabilities compliant with RoHS and REACH regulations are becoming fundamental requirements.
Digital Transformation: Real-time monitoring and analysis of production data through MES systems to enhance transparency and efficiency.
Increased Demand for Small Batches and Rapid Response: The urgent production of devices like forehead thermometers and ventilators during the pandemic highlighted the need for PCBA manufacturers to possess rapid response capabilities. The 8-hour expedited response service introduced by Tortai Technologies Co., Ltd. exemplifies adaptation to this trend.

When selecting a medical PCBA manufacturer, technical capability forms the foundation, the quality system provides the assurance, industry experience is a valuable addition, and service attitude acts as the lubricant for long-term collaboration. The optimal partner is not the lowest-cost supplier, but rather a long-term strategic partner that understands the specificities of the medical industry, possesses professional technical capabilities, and maintains a rigorous quality consciousness.
As demonstrated by the practices of Tortai Technologies Co., Ltd., an excellent medical PCBA manufacturer can provide solid support for the R&D and production of medical device companies through professional certifications, one-stop services, strong production capacity, and efficient expedited response mechanisms. Only by viewing the manufacturer as a strategic partner, rather than merely a supplier, can one gain a competitive edge in the intense medical market.


